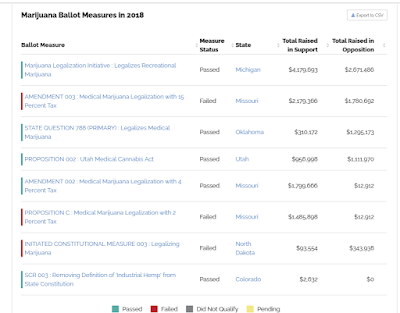
I will be adding more to this blog post in the near future. Here are some Images taken from OpenSecrets.Org on Marijuana Legalization.
Missouri Agribusiness and Farmers MOhemp Energy wants to work with you and is actively seeking: Partners, Investors, Advisers, Team Members, Farmers: who are interested in: Biomass, Biofuels, Hemp Lignin, Energy Conserving Building Products, Hemp Oil, Hemp Fibers, Medical Cannabis, Phytoremediation,
Search This Blog
Sunday, October 16, 2022
Should Cannabis Be Legal Pt 2
Friday, January 8, 2021
Animal Feed Might Just Go to Pot -- And That's a Good Thing
Hemp Industries Association- Anyone who follows the hemp market is aware of how prices have collapsed under the weight of too much supply and not enough demand. But what if demand increased not from new recreational pot users and health conscious consumers, but from animal feed providers?
It might be time to look beyond CBD for hemp stocks as the future may instead be at the feed lots.
States that led in hemp acreage in 2019, including Tennessee, Colorado, and Oregon, saw significant declines in 2020. The states that did see growth were either those that had small pilot projects in 2019 or just started their programs in 2020. https://t.co/u4WmzZKuVl pic.twitter.com/GdKIp6i9Fi
— Hemp Industries Association (@thehia) January 5, 2021
Friday, August 7, 2020
Hemp Farmers Wanted
@PlastiHemp I'm highlighting your company on the MOhemp Blog see Hemp Farmers Wanted https://t.co/6Uun2MCuKG
— Scotty (@StLHandyMan) August 7, 2020
At the Hemp Plastic Company, we turn waste hemp material into Renewable, Sustainable, Compostable, Hemp based Bioplastic Looking
for hemp farmers, processors, and those interested in a better planet. #hemp #hempplastic #hempfarm #compostable #renewable, #sustainable #bioplastic pic.twitter.com/U4cuvKVOKg
— The Hemp Plastic Company (@PlasticHemp) February 16, 2019
We offer a renewable, sustainable, biodegradable, even compostable alternative to fossil fuel based plastic. Since our job is Conversion, tell us! What products would YOU rather buy if they were made from Hemp Plastic? #renewable #hemp #bioplastic #packaging #Manufacturing pic.twitter.com/Ktel0TXrKh
— The Hemp Plastic Company (@PlasticHemp) February 21, 2019
Wednesday, January 30, 2019
WWE Daniel Bryan Shows Off Hemp Belt
Update 3-11 Ronda- Wrestling inst real
— Ryan Satin (@ryansatin) March 11, 2019
Reposted the #WWEhempbelt Video to the "Hemp Environmental Forum" FB page https://t.co/GpLERV0phC— Scotty (@StLHandyMan) January 30, 2019
WWE SmackDown
The "NEW" Daniel Bryan unveils the NEW WWE Championship made from sustainable, organic hemp!
Here's a few more images of the WWE Hemp belt
Tuesday, December 25, 2018
FDA statement on Hemp + Farmbill
Statement
Today, the Agriculture Improvement Act of 2018 was signed into law. Among other things, this new law changes certain federal authorities relating to the production and marketing of hemp, defined as cannabis (Cannabis sativa L.), and derivatives of cannabis with extremely low (less than 0.3 percent on a dry weight basis) concentrations of the psychoactive compound delta-9-tetrahydrocannabinol (THC). These changes include removing hemp from the Controlled Substances Act, which means that it will no longer be an illegal substance under federal law.
Just as important for the FDA and our commitment to protect and promote the public health is what the law didn’t change: Congress explicitly preserved the agency’s current authority to regulate products containing cannabis or cannabis-derived compounds under the Federal Food, Drug, and Cosmetic Act (FD&C Act) and section 351 of the Public Health Service Act. In doing so, Congress recognized the agency’s important public health role with respect to all the products it regulates. This allows the FDA to continue enforcing the law to protect patients and the public while also providing potential regulatory pathways for products containing cannabis and cannabis-derived compounds.
We’re aware of the growing public interest in cannabis and cannabis-derived products, including cannabidiol (CBD). This increasing public interest in these products makes it even more important with the passage of this law for the FDA to clarify its regulatory authority over these products. In short, we treat products containing cannabis or cannabis-derived compounds as we do any other FDA-regulated products — meaning they’re subject to the same authorities and requirements as FDA-regulated products containing any other substance. This is true regardless of the source of the substance, including whether the substance is derived from a plant that is classified as hemp under the Agriculture Improvement Act. To help members of the public understand how the FDA’s requirements apply to these products, the FDA has maintained a webpage with answers to frequently asked questions, which we intend to update moving forward to address questions regarding the Agriculture Improvement Act and regulation of these products generally.
In view of the proliferation of products containing cannabis or cannabis-derived substances, the FDA will advance new steps to better define our public health obligations in this area. We’ll also continue to closely scrutinize products that could pose risks to consumers. Where we believe consumers are being put at risk, the FDA will warn consumers and take enforcement actions.
In particular, we continue to be concerned at the number of drug claims being made about products not approved by the FDA that claim to contain CBD or other cannabis-derived compounds. Among other things, the FDA requires a cannabis product (hemp-derived or otherwise) that is marketed with a claim of therapeutic benefit, or with any other disease claim, to be approved by the FDA for its intended use before it may be introduced into interstate commerce. This is the same standard to which we hold any product marketed as a drug for human or animal use. Cannabis and cannabis-derived products claiming in their marketing and promotional materials that they’re intended for use in the diagnosis, cure, mitigation, treatment, or prevention of diseases (such as cancer, Alzheimer’s disease, psychiatric disorders and diabetes) are considered new drugs or new animal drugs and must go through the FDA drug approval process for human or animal use before they are marketed in the U.S. Selling unapproved products with unsubstantiated therapeutic claims is not only a violation of the law, but also can put patients at risk, as these products have not been proven to be safe or effective. This deceptive marketing of unproven treatments raises significant public health concerns, as it may keep some patients from accessing appropriate, recognized therapies to treat serious and even fatal diseases.
Additionally, it’s unlawful under the FD&C Act to introduce food containing added CBD or THC into interstate commerce, or to market CBD or THC products as, or in, dietary supplements, regardless of whether the substances are hemp-derived. This is because both CBD and THC are active ingredients in FDA-approved drugs and were the subject of substantial clinical investigations before they were marketed as foods or dietary supplements. Under the FD&C Act, it’s illegal to introduce drug ingredients like these into the food supply, or to market them as dietary supplements. This is a requirement that we apply across the board to food products that contain substances that are active ingredients in any drug.
We’ll take enforcement action needed to protect public health against companies illegally selling cannabis and cannabis-derived products that can put consumers at risk and are being marketed in violation of the FDA’s authorities. The FDA has sent warning letters in the past to companies illegally selling CBD products that claimed to prevent, diagnose, treat, or cure serious diseases, such as cancer. Some of these products were in further violation of the FD&C Act because they were marketed as dietary supplements or because they involved the addition of CBD to food.
While products containing cannabis and cannabis-derived compounds remain subject to the FDA’s authorities and requirements, there are pathways available for those who seek to lawfully introduce these products into interstate commerce. The FDA will continue to take steps to make the pathways for the lawful marketing of these products more efficient.
These pathways include ways for companies to seek approval from the FDA to market with therapeutic claims a human or animal drug that is derived from cannabis. For example, in June 2018, the FDA approved a drug, Epidiolex, that contains cannabis-derived CBD for the treatment of seizures associated with two rare and severe forms of epilepsy. That approval was based on adequate and well-controlled clinical studies, which gives prescribers confidence in the drug’s uniform strength and consistent delivery that support appropriate dosing needed for treating patients with these complex and serious epilepsy syndromes.
In addition, pathways remain available for the FDA to consider whether there are circumstances in which certain cannabis-derived compounds might be permitted in a food or dietary supplement. Although such products are generally prohibited to be introduced in interstate commerce, the FDA has authority to issue a regulation allowing the use of a pharmaceutical ingredient in a food or dietary supplement. We are taking new steps to evaluate whether we should pursue such a process. However, the FDA would only consider doing so if the agency were able to determine that all other requirements in the FD&C Act are met, including those required for food additives or new dietary ingredients.
It should also be noted that some foods are derived from parts of the hemp plant that may not contain CBD or THC, meaning that their addition to foods might not raise the same issues as the addition of drug ingredients like CBD and THC. We are able to advance the lawful marketing of three such ingredients today. We are announcing that the agency has completed our evaluation of three Generally Recognized as Safe (GRAS) notices related to hulled hemp seeds, hemp seed protein and hemp seed oil and that the agency had no questions regarding the company’s conclusion that the use of such products as described in the notices is safe. Therefore, these products can be legally marketed in human foods for these uses without food additive approval, provided they comply with all other requirements and do not make disease treatment claims.
Given the substantial public interest in this topic and the clear interest of Congress in fostering the development of appropriate hemp products, we intend to hold a public meeting in the near future for stakeholders to share their experiences and challenges with these products, including information and views related to the safety of such products.
We’ll use this meeting to gather additional input relevant to the lawful pathways by which products containing cannabis or cannabis-derived compounds can be marketed, and how we can make these legal pathways more predictable and efficient. We’ll also solicit input relevant to our regulatory strategy related to existing products, while we continue to evaluate and take action against products that are being unlawfully marketed and create risks for consumers.
At the same time, we recognize the potential opportunities that cannabis or cannabis-derived compounds could offer and acknowledge the significant interest in these possibilities. We’re committed to pursuing an efficient regulatory framework for allowing product developers that meet the requirements under our authorities to lawfully market these types of products.
The FDA, an agency within the U.S. Department of Health and Human Services, protects the public health by assuring the safety, effectiveness, and security of human and veterinary drugs, vaccines and other biological products for human use, and medical devices. The agency also is responsible for the safety and security of our nation’s food supply, cosmetics, dietary supplements, products that give off electronic radiation, and for regulating tobacco products.
https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm628988.htm?
###
Saturday, June 16, 2018
Missouri Industrial Hemp Law 6-1-2018
@tonymess > @paulcurtman @MoAgriculture @BrianforSenate < the MoLeg Hemp legislation is so short sided. Isn't it the Politicians JOB to Help Missourians? or is it same ole same ole moleg picks the winners and losers? > https://t.co/VneSwEWuqc— Scotty (@StLHandyMan) June 17, 2018
Sunday, September 3, 2017
Hemp Field Day Sep 16, 2017 in Simpsonville, Kentucky
Friday, September 16, 2016
A Plant that could change evrerything
Hemp A Plant that could change everything. Hemp-Kids Get It
 |
| Hemp, Kids Get It DOE Bioenergize Challenge Winners |
Tuesday, May 10, 2016
Hemp: A Source Biomass Antibacterial Fibers
Cannabis sativa: The Plant of the Thousand and One Molecules
Plant lignocellulosic biomass is an abundant renewable resource, which can provide biopolymers, fibers, chemicals and energy (Guerriero et al., 2014, 2015, 2016).
Hemp Stem: A Source of Fibers with Antibacterial Properties
REVIEW ARTICLE
 Christelle M. Andre*,
Christelle M. Andre*,  Jean-Francois Hausman and
Jean-Francois Hausman and  Gea Guerriero
Gea Guerriero
- Environmental Research and Innovation, Luxembourg Institute of Science and Technology, Esch-sur-Alzette, Luxembourg
Tuesday, April 19, 2016
International Hemp Environment Forum Kyoto Event
「第1回世界麻環境フォーラム」は2016年7月2日、
2回目以降は開催地国を変えて国際年次イベントとする予
ネットワークは世界中の、まさに5大陸をまたいだ参加者
今年の1月に日本を起点に動きが始まり繋がったFace
「世界麻環境フォーラム」Hemp Environmental Forum の結成の目的は「持続可能な未来の創造」です。その為に
達成目的として「国際麻環境基金」の立上げを視野に入れ

This is a whole earth project, from 5 continents, covering earth. No matter in which region or country you live, what jobs or business you are doing, rich or poor or whatsoever, global climate change you are facing to is real. As long as we depends on toxic energy that is killing us, there is no sustainable future. It's time to be aware that Hemp is the champion biomass with its versatile usage, it's so versatile that it can substitute to fossil fuel which causes a lot of environmental problems and also substitute to precious forests which are giving us oxygen that all the animals on earth need by all means. Destruction of forests are exigency level too.
With the new sophisticated ideas and innovated techniques combined with those traditional knowledge and wisdom towards sustainability, we can really implement hemp farming and its utility and can realize sustainable future which is talked in a book "HEMP Lifeline to the Future". This is the matter of survival and hope for all of us living on earth which is a planet with limited resources human can utilize. She can feed all of us if we plow the land and cultivate plants and use it humbly as renewable resource, and we can share all. We get unite, be aware, share the info and knowledge and implement together with worldwide network and cooperation with compassion to each other. It's time to start. We wish 2nd July 2016 in Kyoto will be memorized in a history that we remember the way how to keep surviving all of us with renewable natural resources, specially cannabis HEMP as a symbol of biomass and earth's precious gift we can use for ourselves and for our earth to be healed.
Registered Non Profit Organization (NPO), Japan Hemp Association
together with Hemp Environmental Forum, international network
Saturday, January 16, 2016
Hemp Environmental Forum
Melissa Balin added a new photo to Hemp Environmental Forum's timeline —
#HempHempHooray! I am the second like at http://www.facebook.com/HempEnvironmentalForum -where is everybody? Could you please share any of the informative and very important links you have shared in this thread there so that we have shareable links that people will then share from their own pages that will draw more actualized beings to also like the page and share the posts- also for the purposes of interactivity- and in order to cover the many different aspects of usefulness of the Hemp plant without glossing over some of the lesser appreciated nuances- perhaps Takashi or Nayer might propose a weekly topic or issue to explore and everyone can weigh in with opinions and insights... I am partial to #Hemp 4 #Phytoremediation and everyone who knows me knows that I spend a lot of my personal research in California appreciating Hemp as a medicine... I think a weekly topic for discussion such as #DidYouKnow... #Hemp is for Building with a link to the research and use of Hempcrete etc. and then Hempsters around the globe can share their links and feedback each week- #DidYouKnow #Hemp is for #Eating, etc. just trying to think of a way for this page to be different than other fan pages in terms of aggregating new content and culling it in a meaningful way for sharing purposes - like a syllabus or living open source workbook of sorts for the year- particularly because I have found that there is some really radical research already existing about issues like whether or not Cannabis can eradicate cancers and studies in the Czech and Israel that are simply not being shared or cited in other countries so it would be nice to bridge that gap with a continuing dialogue- Keep up the great work everybody!
Hemp Environmental Forum
Hemp Environmental Forum